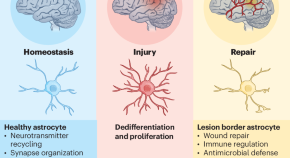

Astrocytes have important roles in the repair of the CNS. However, the underlying mechanisms involved remain incompletely understood. O’Shea et al. report that the functional reprogramming of astrocytes at the borders of traumatic lesions contributes to the re-establishment of CNS integrity by separating the parenchyma from stromal and immune cells.
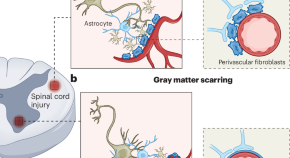
After spinal cord injury, stromal fibroblasts originate from pericytes and perivascular fibroblasts, with pericytes more prevalent in gray matter and fibroblasts in white matter. Holl et al. show that both cell types respond to injury and inflammation, are activated, and transcriptionally converge on scar formation after injury, paving the way for therapeutic possibilities.
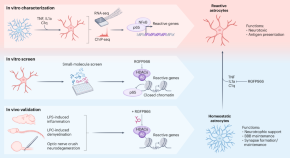
Astrocytes have important roles in disease and are difficult to modulate, owing to a paucity of known targets. Clayton et al. develop a screening platform to unbiasedly identify modulators of astrocyte reactivity. They discover that HDAC3 inhibitors regulate astrocyte transitions into their reactive phenotype in vitro and in vivo.
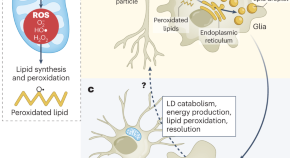
The Sehgal lab presents data showing that the non-cell autonomous pathway of glial lipid droplet formation occurs during sleep and helps to resolve neuronal reactive oxygen species (ROS). This promotes neuronal function after an active day. Hence, this pathway has an important physiological function beyond its previously described role in ROS-associated diseases, including Alzheimer’s disease.
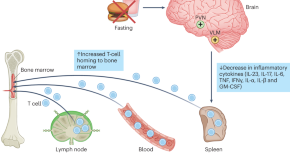
Both caloric restriction and obesity affect autoimmune diseases. The activation of brainstem neurons in the ventrolateral medulla (VLM) with fasting suppresses experimental autoimmune diseases. Stimulation of VLM neurons alters T cell traffic by redistributing immune cells to bone marrow and reduces inflammatory cytokine production, thus providing therapy of experimental autoimmunity.
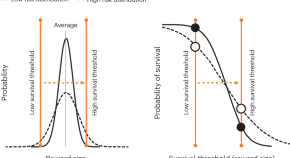
Muller et al. demonstrate that reward signals recorded from the frontal cortex of nonhuman primates exhibit a population-based scheme for learning probability distributions over reward values. This study provides evidence that neural signals outside of the midbrain reflect the principles of distributional reinforcement-learning theory.
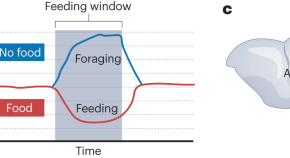
Neuroscientists have long believed that hunger increases activity in agouti-related peptide (AgRP) neurons to regulate feeding-related behaviors and metabolism, but a new study shows that the story is much more complicated. Sayar-Atasoy and colleagues show that the time of day uncouples activity in AgRP neurons from hunger and demonstrate how daily feeding patterns influence future AgRP neuron activity.
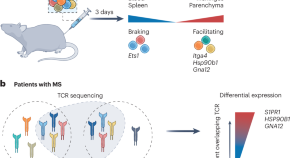
Multiple sclerosis (MS) is an autoimmune disease of the central nervous system that is driven by autoreactive lymphocytes. An in vivo CRISPR screen of T cell infiltration in a rat model of MS now identifies the genetic modules that control this key step in the immunopathology of MS.
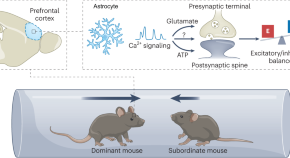
Noh et al. show that the regulation of social behavior extends beyond neurons. Astrocytes, a major type of glial cell in the CNS, can influence social dominance behavior by modulating excitatory and inhibitory neural activities in the dorsomedial prefrontal cortex of adult male mice. This work highlights the importance of neuron–astrocyte interplay in controlling ethologically relevant behaviors.
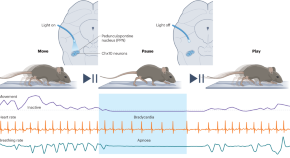
Activating a specific subpopulation of glutamatergic neurons in the brainstem issues a motor command for global motor arrest. This motor arrest is distinct from defensive freezing and has a striking pause-and-play pattern accompanied by a reduction in respiration and heart rate.
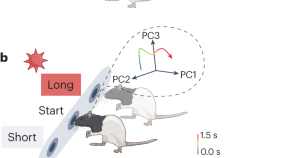
Monteiro and colleagues used temperature manipulation to bidirectionally alter the speed of neuronal dynamics in the dorsal striatum of anesthetized rats. This manipulation selectively slowed down or sped up time perception, providing insights into the mechanisms of time-based decisions.
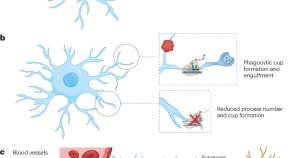
Vasek et al. show that microglia perform protein translation in their processes and that this translation is important for the number of processes and formation of phagocytic cups. These findings may provide insight into how microglia respond rapidly to a wide variety of local signals in defined cellular compartments.
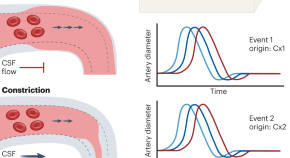
A study by Holstein-Rønsbo, Gan et al. published in this issue of Nature Neuroscience adds another dimension to the ‘glymphatic’ story — the role of functional hyperemia facilitating perivascular flow of cerebrospinal fluid along pial arteries.
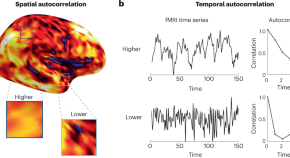
In this issue, Shinn et al. demonstrate a close relationship between complex brain network topology and lower-level statistical properties of neuroimaging data. They also highlight the potential of these statistical measures, which capture similarity in space and time, to provide imaging-based markers of aging and pharmacological states.

Zhu et al. show that basal forebrain cholinergic neurons send fast, specific information about a broad range of sensory stimuli to the auditory cortex, modulated by slower fluctuations in cholinergic activity. These findings help us to understand how the cholinergic system multiplexes diverse representations to fulfil its multifaceted roles in attention, learning and memory.
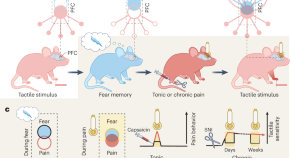
Stegemann et al. have unraveled a long-lasting role for fear engrams in future pain perception. Prefrontal pain and fear representations become entangled following a fearful event. As subsequent painful experiences also reactivate the fear network, silencing the fear engram alone can alleviate both tonic pain and hypersensitivity in chronic pain.
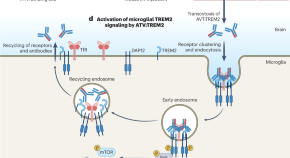
Loss-of-function variants of TREM2 increase risk for Alzheimer’s disease. A new study presents a therapeutic candidate — ATV:TREM2, a TREM2-activating antibody engineered with a transferrin receptor binding site to facilitate blood-to-brain transport. Treatment of a mouse model of Alzheimer’s disease with ATV:TREM2 improved energy metabolism and microglial function.
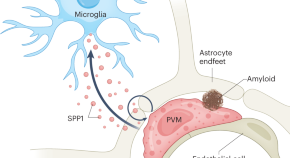
Microglial synapse engulfment precedes brain amyloid plaque formation and probably contributes to early cognitive decline in Alzheimer’s disease. The mechanisms that regulate microglia-mediated synapse engulfment are unclear. De Schepper et al. show that perivascular SPP1 induces microglia-mediated synapse engulfment, highlighting a neuroimmune interaction that contributes to synapse loss in amyloid pathology.
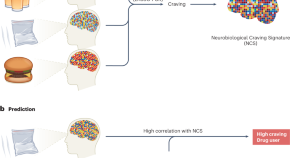
Craving is a core characteristic of drug addiction and eating disorders. A new study identifies an fMRI-based neural signature of craving that is common to both food and drugs, predicts self-reported craving, distinguishes drug users from non-users, and tracks the efficacy of a cognitive therapy technique to reduce craving.
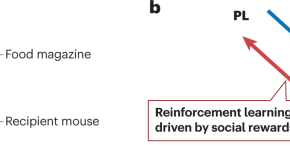
Scheggia et al. 1 have established a behavioral paradigm to explore preferences for ‘altruistic’ or ‘selfish’ choice behavior in mice. The results suggest that altruistic behavior develops through reinforcement learning driven by social rewards, which is controlled by interactions between the basolateral amygdala and prelimbic cortex.